In the following pages, we intend to provide enough information for you to begin creating a stockpiling system that will accomplish those goals, whether the infectious outbreak risk is
- seasonal flu
- pandemic influenza
- a unique pathogen (such as Ebola virus).
Before you begin stockpiling PPE, there are ten important questions you should ask.
1. How are seasonal flu and pandemic flu different?
According to the CDC1, influenza B and some influenza A viruses are easily transmitted among humans and cause seasonal epidemics.
Influenza A viruses infect many different animals, as well as humans. An influenza pandemic can be caused by the emergence of a new and different influenza A virus:
- Which has the ability to create sustained human-to-human transmission and
- For which the human population has little to no immunity.
To learn more click here:
www.cdc.gov/flu/about/viruses/types.htm
Halyard has researched the available material to bring you an overview of PPE Guidelines for Pandemic Preparedness from the Occupational Safety & Health Administration (OSHA), the Centers for Disease Control and Prevention (CDC), and the World Health Organization (WHO), as of 2018. We’ve also provided links for more detailed information.
2. What types of PPE do we need to prepare for a seasonal flu outbreak?
Whenever you enter a flu patient’s room, use a surgical mask to protect yourself from contact with the large droplets generated when a patient coughs, sneezes, talks, or breathes.2FDA-approved surgical masks come in many styles, including styles with ear-loops, ties, and with or without protective eye shield extensions.
If you are going to be performing aerosol-generating procedures, use a respirator and goggles; at minimum, use a fit-tested N95 disposable respirator. For maximum protection, use a full face shield in front of a respirator.
www.osha.gov/dts/guidance/flu/healthcare.html
For any tasks that might create splashes or otherwise put you at risk of contamination, wear gloves, gowns, and eye protection. And be sure you don and doff your PPE in the correct order to avoid contact with infectious elements.3
www.osha.gov/dts/guidance/flu/protectyourself_healthcare.html
3. How much PPE should we stockpile for a seasonal flu outbreak?
When calculating stockpile requirements for the flu season ahead, it’s helpful to ask the following questions:
What PPE products will most likely have increased demand during an intense flu season?
- Ear-loop or tie masks?
- Face shields?
- Isolation or surgical gowns?
- Exam gloves?
- Other PPE in your facility’s protocol?
Who will wear the PPE when encountering flu patients?2
- Doctors/Nurses?
- Patients?
- Visitors?
- Other Staff (EMS, Lab, Transport, Environmental)?
You can also find guidance4 at the following link:
Related Article
4. What types of PPE do we need to prepare for a pandemic flu outbreak?
The types of PPE appropriate for pandemic flu are similar to those for seasonal flu, including surgical masks, face shields, isolation or surgical gowns, and exam gloves (see Question 2, above). Additionally, respiratory protection (e.g. N95 surgical respirators) may be a key component of your pandemic flu PPE protocols. Compared to seasonal flu, more staff and tasks may be considered high risk in pandemic flu.
OSHA does make a distinction4 regarding gowns when caring for pandemic flu patients:
Wear an isolation gown when you are at risk of soiling your clothes or uniform with blood or other bodily fluids, including respiratory secretions. However, Health and Human Services (HHS) states that most routine pandemic influenza patient encounters don’t require the use of gowns. Examples of when a gown may be needed include procedures such as intubation or when closely holding a pediatric patient. Gowns should fully cover the areas of your body requiring protection.
Important Tip: Research your current PPE manufacturer’s supply chain. The farther away these items are produced, the longer it will take to fill large emergency orders. HALYARD* face masks and respirators are primarily manufactured in North America, which enables us to respond faster in emergencies and outbreaks
5. How much PPE should we stockpile for a pandemic flu outbreak?
In pandemic flu, the quantity of PPE required may be significantly more than seasonal flu. More employees may be considered at very high or high risk. Also, the duration of a pandemic may be longer than seasonal flu. OSHA suggests acommunity can assume two waves of a pandemic, each 12 weeks (24 weeks total).5
The US Department of Labor (DOL) and HHS encourage employers to stockpile facemasks and respirators in advance of an influenza pandemic. Manufacturing capacity at the time of an outbreak would not meet expected demand during the pandemic. HHS estimates that a pandemic outbreak would require at least 90 million N95 respirators in just a 42-day period.6
According to OSHA, the hospital should assess the occupational exposure risk for each job (not all hospital employees provide patient care) and work task (e.g., aerosol-generating medical procedures). Categorize risks as very high, high, medium, and low, then estimate the number or percentage of employees who fall into each category to estimate the types and amounts of PPE you need.5
6. How should we prepare for a pandemic involving a unique pathogen such as the Ebola virus?
It is impossible to prepare for pandemics caused by every conceivable unique pathogen, but there are some guidelines for the Ebola virus (EVD) that may apply in other situations.7 For example, the Tiered Approach classifies each hospital by the role they will play in a pandemic:
- FRONTLINE hospital –Most acute care acute care hospitals, urgent care clinics and critical access hospitals
- ASSESSMENT hospital –facilities prepared to receive, isolate, and care for a Person Under Investigation (PUI) until a diagnosis can be confirmed or ruled out.
- TREATMENT hospital –prepared to care for patients with confirmed EVD throughout the course of their illness.
For more about the Tiered Approach, see
https://www.cdc.gov/vhf/ebola/healthcare-us/ppe/supplies.html
7. What types of PPE should be used in outbreaks of unique pathogens such as Ebola virus?
Based on experience with the Ebola outbreak, the CDC provides recommendations for appropriate PPE depending on the status of the patient:
For managing patients who are under investi-gation (PUI) for Ebola who are clinically stable and do not have bleeding, vomiting or diarrhea:7
- Single-use (disposable) fluid-resistant gown† that extends to at least mid-calf or fluid-resistant coveralls without an integrated hood
- Single-use full face shield
- Single-use face mask
- Single-use exam gloves with extended cuffs. Two pairs of gloves should be worn. At a minimum, outer gloves should have extended cuffs
† NOTE: In section 6 of the CDC Guideline “Selecting PPE for Healthcare Workers Who Care for Patients with Ebola”, impermeableindicates that the material and construction have demonstrated resistance to synthetic blood andsimulated bloodborne pathogens. In contrast, fluid-resistant indicates a gown that has demonstrated resistance to water or a coverall that has demonstrated resistance to water or synthetic blood.7
For details on the required testing for fluid resistance and resistance to bloodborne pathogens, see Table 1 in the following:
www.cdc.gov/vhf/ebola/healthcare-us/ppe/guidance-clinically-stable-puis.html
For managing patients with confirmed Ebola who are clinically unstable or have bleeding, vomiting or diarrhea.7
- Single-use (disposable) impermeable gown† extending to at least mid-calf, Single-use impermeable coverall. (preferably without an integrated hood).
- Respiratory Protection: In case a potentially aerosol-generating procedure needs to be performed emergently, use either
- a PAPR (a hooded respirator with a full face shield, helmet, or headpiece) covered with a disposable hood, or
- a disposable, NIOSH-certified N95 respirator mask combined with single-use surgical hood and a single-use full face shield.
- Disposable exam gloves with extended cuffs. Two pairs of gloves should be worn so that a heavily soiled outer glove can be safely removed and replaced during care.
- Single-use boot covers that extend to at least mid-calf. In addition, single-use ankle-high shoe covers (“surgical booties”) worn over boot covers may be considered to facilitate the doffing process.
- Disposable apron that covers the torso to the level of the mid-calf should be used over the gown or coveralls
NEED HELP ESTIMATING STOCKPILE QUANTITIES?
To help you estimate PPE requirements for seasonal flu and pandemic events, contact your Halyard representative.
NOTE: CDC Guidance on Ebola virus also highlights the need for a trained observer to ensure the PPE protocol is adhered to properly, including proper donning and doffing of the PPE ensemble.
Based on existing OSHA and CDC guidance, OSHA has developed this detailed PPE Selection Matrix to help employers select appropriate PPE for workers who may be exposed to unique pathogens such as the Ebola virus.8
OSHA PPE SELECTION MATRIX FOR OCCUPATIONAL EXPOSURE TO EBOLA VIRUS
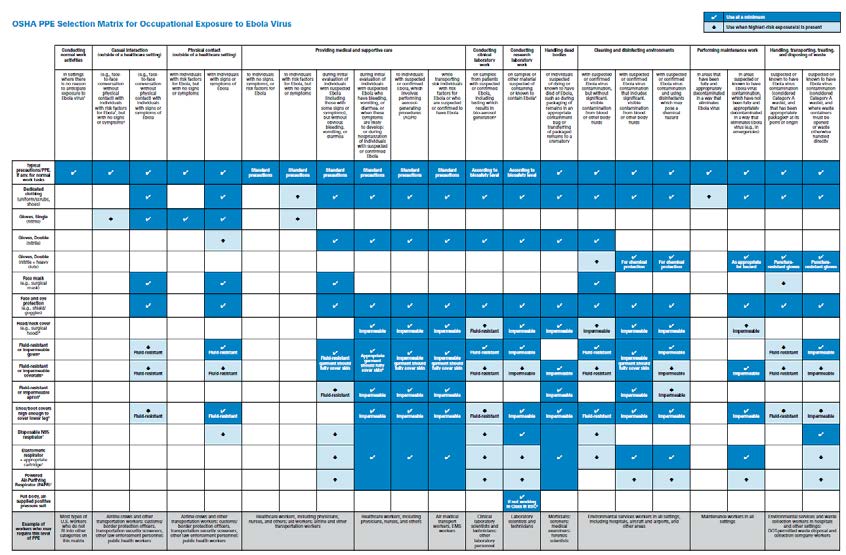
8. How much PPE should we stockpile for a pandemic caused by a unique pathogen?
Begin with the guidelines for the role your hospital will play. In the Tiered Approach, the duration of exposure and type of exposure will influence your PPE requirements.6
- FRONTLINE hospital –PPE sufficient for 12 to 24 hours of care
- ASSESSMENT hospital –PPE sufficient for 4 to 5 days of care
- TREATMENT hospital –PPE sufficient for 7 days
According to the CDC, the amount of PPE needed for each day of patient care will depend on various factors including:
- Expected number of patients
- Acuity of patients
- Projected number of staff
- Healthcare team configuration
- Length of shifts
- Number of required breaks for staff wearing PPE
Other factors include isolation unit location and staff support strategies, waste management strategy, lab location, lab testing demand, and hospital protocols for products.6
THE IMPORTANCE OF CUSTOMIZING YOUR PREPAREDNESS PLAN
In the event of a major outbreak, effective vaccines may not be available until 4 to 6 months into the pandemic, and shortages of antiviral medications could also occur, making it especially important to have the necessary PPE available to protect healthcare workers.
One study9detailed the impact of the SARS outbreak in Toronto, which lasted approximately 6-7 months. (There were 438 confirmed or suspected SARS cases in Canada.) One large hospital reported that at the height of the epidemic the daily consumption of PPE equipment included 3,000 disposable gowns, 14,000 pairs of gloves, 18,000 N95 respirators, 9,500 ear loop masks, and 500 pairs of goggles. In the first week of the SARS outbreak, the hospital purchased
One study9detailed the impact of the SARS outbreak in Toronto, which lasted approximately 6-7 months. (There were 438 confirmed or suspected SARS cases in Canada.) One large hospital reported that at the height of the epidemic the daily consumption of PPE equipment included 3,000 disposable gowns, 14,000 pairs of gloves, 18,000 N95 respirators, 9,500 ear loop masks, and 500 pairs of goggles. In the first week of the SARS outbreak, the hospital purchased $1 million worth of supplies.
Your facility’s unique pathogen scenarios, scope and duration could differ dramatically, so careful thought should be given to your local and regional risks.
9. Do we need extra PPE for patients and visitors during flu season?
Yes. To help control the spread of flu, facemasks should be worn by patients who are presenting with flu-like symptoms prior to treatment and by patients with a confirmed diagnosis of influenza while they are being transported within the hospital for tests, x-rays, etc.10 Facemasks should also be offered to visitors who are coughing, sneezing, or have other flu-like symptoms. The number of facemasks stockpiled for these purposes should be in addition to the numbers needed for employees’ health and safety.5
For more information, see Interim Guidance for the Use of Masks to Control Influenza Transmission.
www.cdc.gov/flu/professionals/infectioncontrol/maskguidance.htm
10. How should we manage our stockpile once it’s established?
- Prevent damage, contamination, spoilage, or disappearance of supplies. Place stockpiles in clean, secure environments with environmental controls and avoid storage areas that are damp or have temperature extremes.5
- When stockpiling items, be aware of each product’s shelf life and storage conditions. Where possible, incorporate product rotation (consume the oldest supplies first) in your stockpile management system.5
- Consider a regional plan for PPE – Hospitals are encouraged to partner with neighboring hospitals, in-network facilities, and/or healthcare coalitions to share inventory for limited supply products. Understand how quickly manufacturers and distributors can respond to ramp up critical supplies.6
- Keep training current – Ensure critical employees are trained and regularly practices on donning and doffing procedures. Give special consideration to training and use of PPE for unique pathogens and fit-tested respirators, and donning and doffing procedures with and without assistance of a trained observer.5
- https://www.osha.gov/dsg/guidance/proposedGuidanceStockpilingRespirator.pdf
FACE MASK OR RESPIRATOR: WHICH DO YOU NEED?
Face masks are used as a physical barrier to protect you from hazards such as splashes of large droplets of blood or body fluids. Face masks also prevent contamination by trapping large particles of body fluids that may contain bacteria or viruses when they are expelled by the wearer (for example, through coughing or sneezing).
Respirators are used to reduce your exposure to airborne contaminants. Most respirators are designed to fit the face and to provide a tight seal between the respirator’s edge and the face. A proper seal between your face and the respirator forces inhaled air to be pulled through the respirator’s filter material and not through gaps in the seal between your face and the respirator.
A ‘fit test’ is necessary for most models of respirators to ensure that a proper seal can be established between the respirator and your face. (Halyard provides Qualitative Fit Testing kits and information for healthcare: Halyard Qualitative Fit Test)5
For a table comparing the advantages and disadvantages of Respirators and other facemasks, see:
www.osha.gov/dsg/guidance/proposedGuidanceStockpiling Respirator.pdf
Sources
- “Types of Influenza Viruses”, in section “Understanding Flu Viruses” on CDC.gov website. www.cdc.gov/flu/about/viruses/types.htm
- “Employer Guidance Reducing Healthcare Workers’ Exposures to Seasonal Flu Virus” located on the “Seasonal Flu” page of OSHA.gov website. www.osha.gov/dts/guidance/flu/healthcare.html
- “Worker Guidance Precautions for Healthcare Workers during Flu Season” located on the “Seasonal Flu” page of OSHA.gov website. www.osha.gov/dts/guidance/flu/protectyourself_healthcare.html
- “Frequently Asked Questions on Pandemic Influenza Preparedness and Response Guidance for Healthcare Workers and Healthcare Employers” located in the “Pandemic Influenza” section of the OSHA.gov web site. www.osha.gov/SLTC/pandemicinfluenza/pandemic_health.html
- “Proposed Guidance on Workplace Stockpiling of Respirators and Facemasks for Pandemic Influenza” located on OSHA.gov web site. www.osha.gov/dsg/guidance/proposedGuidanceStockpilingRespirator.pdf
- “Considerations for U.S Healthcare Facilities to Ensure Adequate Supplies of Personal Protective Equipment (PPE) for Ebola Preparedness” located in the “Ebola” section of the CDC.gov web site. https://www.cdc.gov/vhf/ebola/healthcare-us/ppe/supplies.html
- “For U.S. Healthcare Settings: Donning and Doffing Personal Protective Equipment (PPE) for Evaluating Persons Under Investigation (PUIs) for Ebola Who Are Clinically Stable and Do Not Have Bleeding, Vomiting, or Diarrhea” located in the “Ebola” section of the CDC.gov web site. www.cdc.gov/vhf/ebola/healthcare-us/ppe/guidance-clinically-stablepuis. html
- OSHA.gov, PPE Selection Matrix for Occupational Exposure to Ebola Virus. www.osha.gov/Publications/OSHA3761.pdf.
- OSHA.gov,“Pandemic Influenza Preparedness and Response Guidance for Healthcare Workers and Healthcare Employers,”, Personal Protective Equipment section,
- “Interim Guidance for the Use of Masks to Control Influenza Transmission”, located on the “Seasonal Influenza” page of the CDC.gov web site. www.cdc.gov/flu/professionals/infectioncontrol/maskguidance.htm
